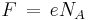Faraday constant
In physics and chemistry, the Faraday constant (named after Michael Faraday) is the magnitude of electric charge per mole of electrons.[1] It has the currently accepted value
- F = 96,485.3365(21) C/mol.[2]
The constant F has a simple relation to two other physical constants:
where:
NA is the Avogadro constant (the ratio of the number of particles 'N' to the amount of substance 'n' - a unit mole), and e is the elementary charge or the magnitude of the charge of an electron. This relation is true because the amount of charge of a mole of electrons is equal to the amount of charge in one electron multiplied by the number of electrons in a mole.
The value of F was first determined by weighing the amount of silver deposited in an electrochemical reaction in which a measured current was passed for a measured time, and using Faraday's law of electrolysis.[5] Research is continuing into more accurate ways of determining the interrelated constants F, NA, and e.
Contents |
Other Common Units of Faraday's Constant
- 96.485 kJ per volt gram equivalent
- 23.061 kcal per volt gram equivalent
- 26.801 A*h/mol
Faraday unit of charge
Related to Faraday's constant is the "faraday", a unit of electrical charge. It is much less common than the coulomb, but sometimes used in electrochemistry.[6] One Faraday of charge is the magnitude of the charge of one mole of electrons, i.e. 96,485.3365(21) C.[2]
Expressed in faradays, the Faraday constant F equals "1 faraday of charge per mole".
This faraday unit is not to be confused with the farad, an unrelated unit of capacitance.
See also
- Farad, unit of capacitance
- Michael Faraday
- Faraday cage
- Faraday efficiency
- Faraday's law of electrolysis
- Faraday's law of Electromagnetic induction
References
- ^ The term "magnitude" is used in the sense of "absolute value": The charge of an electron is negative, but F is always defined to be positive.
- ^ a b "CODATA Value: Faraday constant". The NIST Reference on Constants, Units, and Uncertainty. US National Institute of Standards and Technology. June 2011. http://physics.nist.gov/cgi-bin/cuu/Value?f. Retrieved 2011-06-23.
- ^ "CODATA Value: elementary charge". The NIST Reference on Constants, Units, and Uncertainty. US National Institute of Standards and Technology. June 2011. http://physics.nist.gov/cgi-bin/cuu/Value?e. Retrieved 2011-06-23.
- ^ "CODATA Value: Avogadro constant". The NIST Reference on Constants, Units, and Uncertainty. US National Institute of Standards and Technology. June 2011. http://physics.nist.gov/cgi-bin/cuu/Value?na. Retrieved 2011-06-23.
- ^ NIST Introduction to physical constants
- ^ Foundations Of Physics, Volume 2, by R. S. Gambhir, p51